PROZIS - 12 x H2O Infusion 9 g - Ananas - Ta boisson hydratante préférée, enrichie en vitamine C - Cdiscount Sport
the s†an dard enthalpy of formation of gaseous H2O at 298K is 241.82KJ/mol. Estimatr its value of 373K given the following values of the molar heat capacities at cons†an t pressure: H2O(g):35.58J/Kmol,

Consider the following reaction:{H}_{2}O(l)rightarrow {H}_{2}O(g);Delta {H}_{1}=44kJ2{CH}_{3}OH(l)+3{O}_{2}rightarrow 4{H}_{2}O(l)+2{CO}_{2}(g); Delta {H}_{2}=-1453kJWhat is the value of Delta H the second reaction water vapor is formed instead of ...
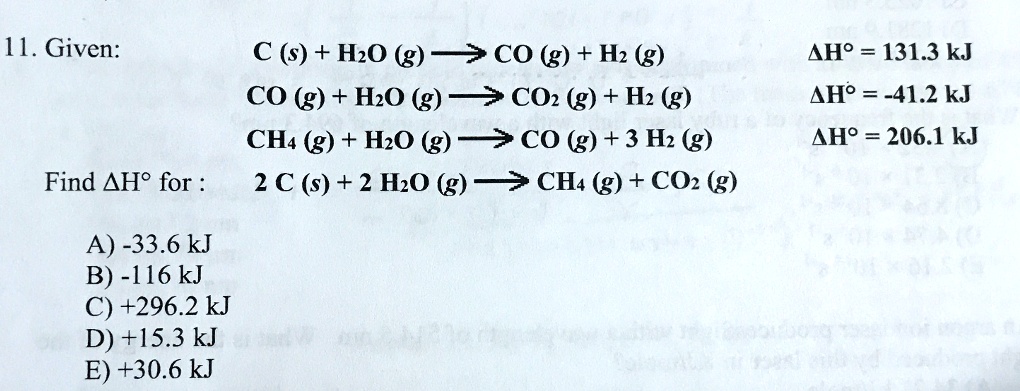
SOLVED: Given: C (s) + H2O (g) -> CO (g) + H2 (g) CO (g) + H2O (g) -> CO2 (g) + H2 (g) CH4 (g) + H2O (g) -> CO (g) +