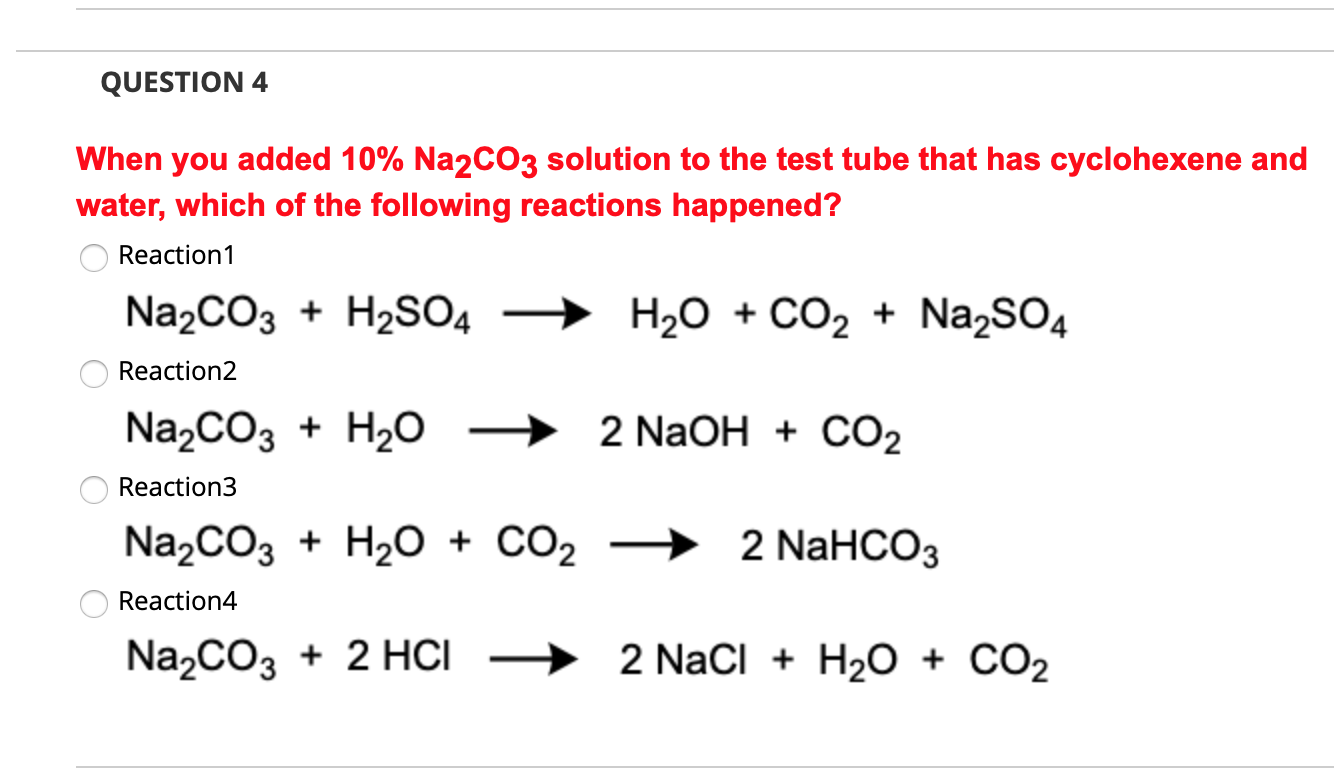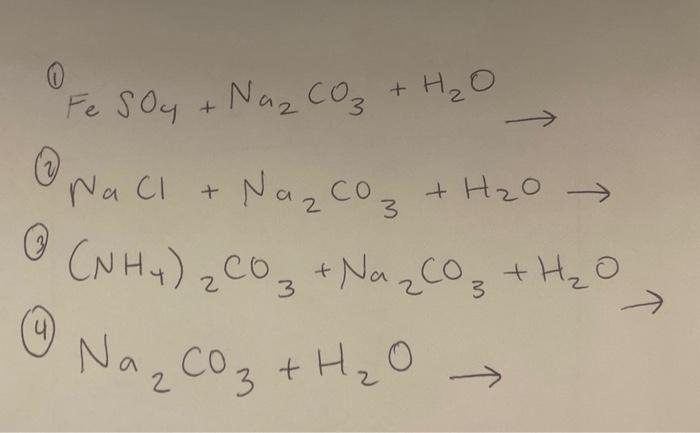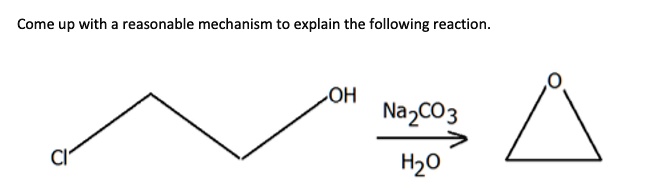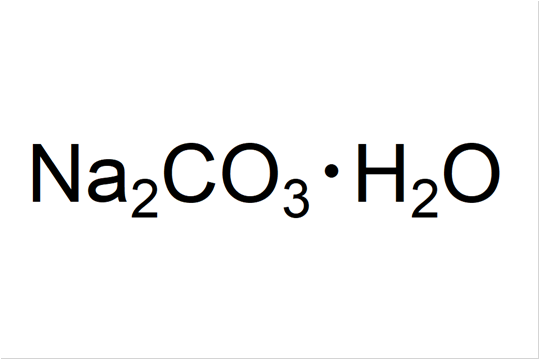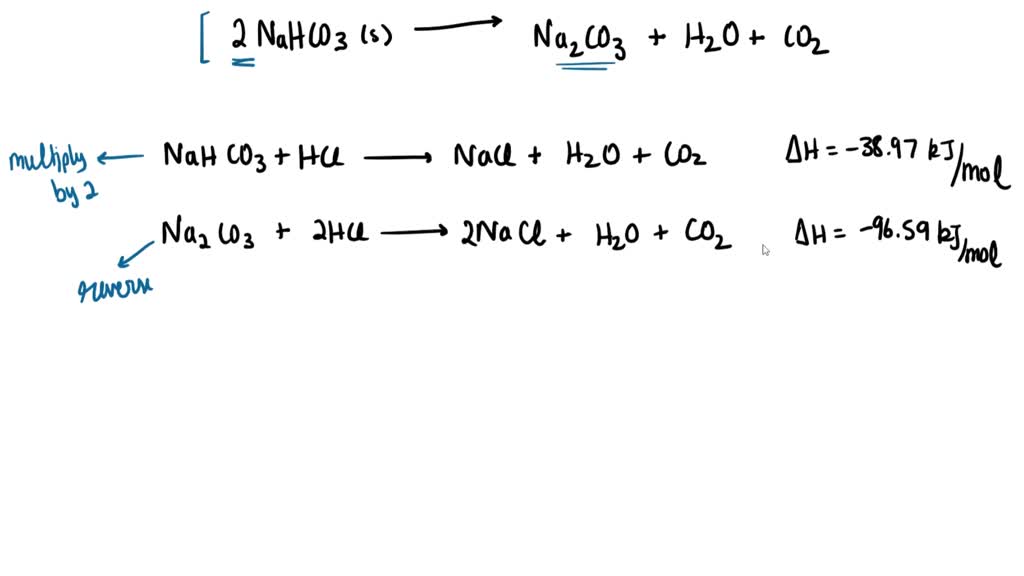
SOLVED: The following data are needed for this question: NaHCO3 (s) + HCl (aq) â†' NaCl (aq) + H2O (l) + CO2 (g) ΔH = -38.97 kJ mol-1 Na2CO3 (s) + 2HCl (

Figure 3 from Solubility equilibria. From data optimization to process simulation | Semantic Scholar

How many mL of 0.1 M HCl are required to react completely with 1 g mixture of Na2CO3 and NaHCO3 containing equimolar amounts of b… | Test tube, Solutions, Completed

The Facile Hydrolysis of Imidazolinium Chlorides (N‐Heterocyclic Carbene Precursors) Under Basic Aqueous Conditions - Touj - Chemistry – A European Journal - Wiley Online Library

Scheme 3. Reagents and conditions: a) H2O, Na2CO3, rt, 1 min; b) CH3CN,... | Download Scientific Diagram